- Overview
- Details & Specs
- Related
Electric Pulse Device Factory - SDZ-II Low Frequency Treatment
-
US$ 39
≥50
-
US$ 45
10–49
-
US$ 48
5–9
-
US$ 50
2–4
Product Attribute
1 years
Product information










Basic Information
- Total Capitalization: Below US$1 Million
- Year Established: 2022
- Total Employees: 11 - 50 People
- Product Certificate: NA
- Business Type: Trading Company
Trading Capabilities
- Total Annual Revenue: Below US$1 Million
- Export Percentage: NA
- OEM Services: NA
- Small Orders Accepted: NA
- Brand Names: NA
- Payment Terms: NA
- Main Competitive Advantages: NA
- Major Customer: NA
- Export Markets: NA
Latest Products from this Supplier
-
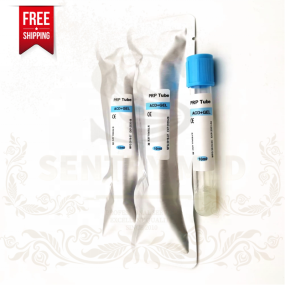
 PRP Tube Manufacturer - ACD Gel Platelet Rich Plasma Bulk
PRP Tube Manufacturer - ACD Gel Platelet Rich Plasma Bulk -
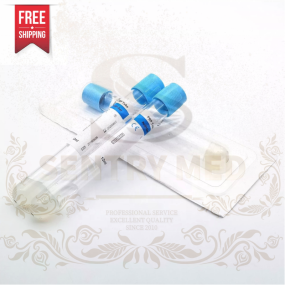
 PRP Tube Supplier - 15ml ACD Gel Color Package Custom
PRP Tube Supplier - 15ml ACD Gel Color Package Custom -
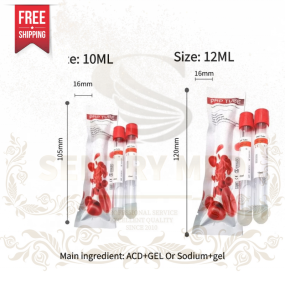
 PRP Tube Factory - 12ml Regen Lab Type Bulk Wholesale
PRP Tube Factory - 12ml Regen Lab Type Bulk Wholesale -
 PRP Tube Manufacturer - ACD-A Without Gel Low Price OEM
PRP Tube Manufacturer - ACD-A Without Gel Low Price OEM -
 PRP Tube Supplier - 12ml Sodium Citrate Gel Free Shipping
PRP Tube Supplier - 12ml Sodium Citrate Gel Free Shipping -
 PRP Tube Factory - Pyrogen Free HA Hyaluronic Acid Custom
PRP Tube Factory - Pyrogen Free HA Hyaluronic Acid Custom -
 Calcium Chloride Manufacturer - 1ml PRP Activator Face Treatment
Calcium Chloride Manufacturer - 1ml PRP Activator Face Treatment -
 PRP Activator Supplier - 1ml Wholesale Price Bulk Supply
PRP Activator Supplier - 1ml Wholesale Price Bulk Supply -
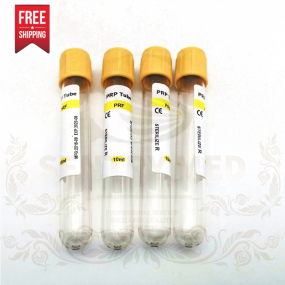
 PRF Tube Manufacturer - 10ml No Additive Centrifuge Custom
PRF Tube Manufacturer - 10ml No Additive Centrifuge Custom -
 PRF Tube Factory - Platelet Rich Fibrin Beauty Red Cap
PRF Tube Factory - Platelet Rich Fibrin Beauty Red Cap